Counting microalgae
A Neubauer haemocytometer can be used for counting microalgae cells with diameters ranging from 2 to 20 mm and at densities up to 500 million cells/ml.
This device consists of a thick rectangular slide with an H-shaped trough delimiting two counting areas. With its special cover slip in place, each area forms a chamber 0.1 mm deep. The total area of each chamber is 9 mm2. Each chamber is divided into 1 mm2 squares: the four corner squares are subdivided into 16 smaller squares, whereas the central one is subdivided into 25 smaller squares, 0.2 x 0.2 mm, each with an area of 0.04 mm2.
To count cells in a sample, proceed as follows:
1. take a 5-ml sample from each culture and place each sample in a separate test tube. Add one drop of Lugol solution and mix well;
2. prepare clean Neubauer slides and covers;
3. put one drop of well mixed algal suspension on each Neubauer chamber and cover with its cover slip;
4. check under low magnification that the algal cells are evenly distributed: avoid the presence of air bubbles, over flowing, underfilling and uneven distribution of cells. Allow the cells to settle for 5 minutes before counting;
5. start counting at the top left square and count only those cells which lie within or touch the boundary lines chosen according one of the two possibilities: either left and bottom boundary lines or right and top boundary lines;
6. for cells larger than 6 mm and not too dense, make a total count in each of the four corner squares and in the central square; then repeat this count in the second chamber;
7. for minute cells and denser populations, count the cells in 5 smaller squares in the larger central square, then repeat this count in the second chamber;
8. calculate average cell density as follows: if all cells are counted in individual blocks each with an area of 1 mm2 and a volume of 0.1 mm3, the average cell density expressed as cells/ml is given by the total count divided by the number of blocks and multiplied by 10 000;
9. if all cells in ten (five in upper chamber + five in lower chamber) smaller squares (volume = 0.004 mm3)in the central block are counted, the average cell density is the total count multiplied by 4 000 000 and divided by 10, i.e. the total count multiplied by 400 000;
10. record the count for each sample and introduce it in the algae population growth curve prepared for each culture container.
Mass culture of rotifers
For the breeding of many marine finfish species the rotifer Brachionus plicatilis is, up to now, the only live feed that can be used in their very early larval stages. While not a compulsory choice in seabass feeding, its mass production is required for the successful breeding of gilthead seabream (and of other Sparids, grouper and grey mullet), whose small mouth cannot accept larger preys at the onset of larval feeding. Among other valuable characteristics as live feed for fish, B. plicatilis was also chosen due to the relative easiness to culture it in large scale.
Two main strains are used. The so-called small strain (S-type) and a large strain (L-type), 50% bigger in dry weight than the S-type. The average length of the lorica in the adult S-type rotifers is 130 µm, whereas it is 240 µm in the L-type. The two strains also show different temperature and salinity tolerances. In the last years mainly S-type زrotifers are reared in the hatcheries.
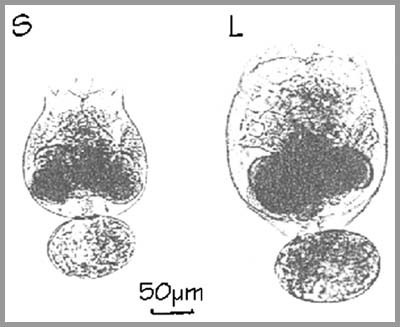
Fig. 26 Brachionus rotundiformis and Brachionus plicatilis (modified from Fu et al.)
Population dynamics
Rotifers can reproduce both sexually (mictic reproduction) and asexually (amictic reproduction), according to the environmental conditions. Usually amictic, rotifers may turn to sexual reproduction when sudden changes in salinity or temperature take place. Then, they produce large resting eggs, similar to brine shrimp cysts. However, in hatcheries is the asexual reproduction that provides the large amounts of animals required for the early feeding of fish larvae. Rotifer population dynamics under mass rearing conditions follow different phases, mimicking those of microalgae:
- the lag-phase, when, just after the inoculum, rotifers begin to consume the phytoplankton of their culture medium and the number of both egg-bearing individuals as well as the quantity of amictic eggs increases;
- the log-phase (or exponential phase), where rotifers reproduce very fast and population growth is exponential;
- the transitional phase (or declining growth), where growth rate slows down and egg-bearing rotifers become rarer;
- the decline phase, where almost only old rotifers without eggs are found and their number decreases rapidly as death rate exceeds growth rate.
The quality of the rotifer population to start new cultures is even more important than in the case of microalgae. To be used as inoculum, the rotifer population must still be in the middle of its log-phase with at least 20% fertility rate (measured as percentage of eggs over total rotifers. Populations in their last declining phase, characterised by limited motility, scarce repletion and absence of egg-bearing animals, should always be discarded. With a proper inoculum and under optimal rearing conditions, a rotifer population should reach its harvesting density within 4 to 5 days.
Under hatchery conditions, rotifer populations can reach the following densities:
in flasks and bags, after 5 to 7 days:
- S-type rotifers, 500 to 700 ind/ml
- L-type rotifers, 150 to 250 ind/ml
in tanks, after 4 to 6 days:
- S-type rotifers, 1000 and more ind/ml
- L-type rotifers, 400 ind/ml
Mass production systems
As this microscopic animal is a filter feeder, its nutritional value strictly depends on its food. In hatcheries, the species is first cultured on microalgae, following the same scale-up protocol described for microalgae, then its final mass production is achieved in large tanks where artificial diets are fed to rapidly increasing numbers, improving at the same time their nutritional value.
The rotifer B. plicatilis is a rather sturdy species able to tolerate a wide range of salinity, temperature and ammonia levels. It can also use several food sources, provided that particle size remains within a 2-20 µm range. Obviously, the highest growth rate is achieved under more restricted environmental parameters, and is closely related to the selected rotifer strain and feeding provided,. In particular, good yields are obtained with high dissolved oxygen levels, temperature at 25°C, pH at 7.5-8.5, salinity in a 20-30 ppt range, less than 1 mg/l free ammonia (NH3), and moderate turbulence. Light is required only when rotifers are fed microalgae.
Dissolved oxygen levels
During the upscaling phase in algal vessels, the oxygen requirements of rotifers are fulfilled by microalgae photosynthetic activity. In mass culture, artificial diets, enrichment boosters, high rotifer densities and metabolic products, contribute to deplete oxygen in the culture medium. To keep its levels within safe margins, i.e. above 80% saturation, a strong aeration is supplied, linked to an emergency oxygen delivery system. See section below for technical details.
Temperature
In mass culture, a temperature as high as 30°C is acceptable, but as bacterial and other contaminants would also increase at these levels, a more manageable 20-ز25°C range is commonly adopted.

Fig. 27 Medium and large volumes cultivated in the same room (photo STM Aquatrade)
pH
An acceptable range is pH 5-9, but the buffer capacity of seawater keeps it within a narrower range. A low pH also influences the balance between the toxic un-ionized ammonia and the ionized form (NH4+). The lower pH also influences the balance between the toxic un-ionized ammonia and the ionized form (NH4 reduces the fraction of toxic NH3.
Salinity
The acceptable salinity range for this rotifer species is quite broad: 1-60 ppt. However there are differences between the two strains, the optimal salinity for the S-type strain being 18-20 ppt, whereas L-type grows better at 30 ppt.
Turbulence
A moderate to strong turbulence is required to keep food particles and rotifers in suspension. The aeration system should provide enough water circulation, but the position of the air diffusers should be adjusted in such a way to avoid stirring and re-suspension of bottom sediments: airstones are hung 15 cm above the bottom, both along the periphery and in the centre of the tanks.
Mass culture facilities for rotifers
Inside the hatchery building, phytoplankton shares the same culture facilities with rotifers, which have a specific section with large tanks for their final mass production. In Mediterranean hatcheries, these tanks have a volume ranging from 1 to 10 m3. These tanks are made of FRP, PE or PVC-lined concrete. As water circulation and particles sedimentation are important in rotifer culture (see below), a widely adopted design is the round tank with conical bottom. This section for mass production of rotifers is equipped with an air distribution system, air conditioning and seawater heaters to keep high water temperatures. Special filtering devices to harvest and rinse rotifers are also available.
As mass culture of rotifers does not require light (except for the phases in which algae are used as food), a normal lighting system is installed. Treated seawater (filtered and sterilized) is provided by the same source that feeds the algal section.
As in microalgae production, hygiene is important and requires the strict implementation of standard cleaning procedures (see Annex 6).
Preparation of the culture medium
The same procedures and precautions described in the algal production section apply to rotifer culture. The only enrichment added to the rotifer culture medium, be it either a log-phase algal culture or treated seawater plus artificial diet, is represented (cyanocobalamin) as a fertility by the addition of vitamin B12 booster for the rotifers. Its dosage is usually 100 ml of B12 stock solution per m3 of rotifer culture in tanks, whereas small vessels and bags are fertilized at the rate of 1 ml/litre. In both cases the vitamin is added together with the inoculum.
To prepare the stock solution put 0.1 g of vitamin B12 into a sterilised 1-l graduated Pyrex bottle and fill with sterilised DW to the mark. When fully dissolved, store in the refrigerator. Warning: never sterilise vitamin solutions.
Pure strain cultures

Fig. 28 Large rotifers tanks for mass culture (photo STM Aquatrade)
The first phases of rotifer cultures (strains and small volume cultures) are performed in the same conditioned room where microalgae are kept, but on separate stands to avoid the risk of possible contamination. The same propagation techniques described for microalgae strains are used for rotifer strains. As for microalgae, culture conditions discourage exponential growth. Rotifer strain inoculums are kept at 1 or 2 individuals per ml in a low-density algal culture and no vitamin is added. Both test tubes and small flasks are used to maintain rotifer strain cultures.
Upscaling rotifer cultures
A clean culture of egg-rich rotifers from a 0.5-l flask is usually inoculated directly into a 5 to 10-l flask, bypassing the 2-l flask stage. The inoculum should provide an initial density of 10 to 20 rotifers per ml. A larger inoculum results in a faster pace of population growth. Rotifers should always be inoculated in algae cultures which have not yet reached their peak growth (that is their log-phase). Environmental conditions should be the same ones at which algae are kept. However, aeration can be reduced to diminish the amount of foam and bottom sediments produced by the metabolic activity of rotifers.
Vitamin B12 is routinely added to all new vessels, at 1 ml of stock solution per litre of algal culture. Mature rotifer cultures from small vessels (5 liters or larger) are poured into bags with an algal population that has not yet entered its full log phase. Five to ten % inoculum is used at this stage, i.e. one 5-liter vessel is used to inoculate one 100-l bag, following the rule of the new culture starting at 30-50 rotifers/ml. Using a sterile volumetric cylinder, add 1 ml of the Vitamin B12 per litre of culture; mark the date on the bag surface, together with the origin of the inoculum and the serial number to facilitate its handling and record keeping.
If the population is growing normally and remains free from contaminants, the horizontal culture method is also frequently used for rotifer culture upscaling. In this case, a good bag makes it possible to inoculate a group of new bags with a initial density of at least 30-50 rotifers/ml.
In the same way as it happens in microalgae cultures, also rotifer cultures are subject to occasional failures (culture crashes). A culture crashes either when rotifers do not multiply as foreseen, or when the entire culture dies abruptly. The possible causes are described below in the Monitoring section. As a precaution against these problems, always maintain a certain number of culture bags in excess of those required by the production schedule. They could be useful to replace quickly acrashed culture at any stage.

Fig. 29 Mature algae bags ready to be inoculate with rotifers (photo STM Aquatrade)
Mass culture
Rotifer mass culture is carried out in large tanks as mentioned above. Because of the very high density achieved (up to 1.000 individuals per ml or more), rearing procedures and protocols to maintain strict hygienic conditions (see Annex 6) have to be rigorously applied. Such routine procedures are described below.
There are basically two main mass rearing methods for rotifers: the older technique that is based on algae and baker’s yeast as food for the rotifers, and the more recent one that uses an artificial diet, the Culture Selco® produced by INVE SA of Belgium (or similar). Both are described below.
Tank preparation
Before starting a new production cycle, normally after the harvest of the previous rotifer culture, prepare the tank as follows:
1. rinse with tap water to eliminate the bulk of organic debris;
2. wash it thoroughly with brush and detergent and rinse it again;
3. wash or spray the tank walls with 500 ppm active chlorine solution (Annex 6); after a couple of hours, drain the tank and rinse it well until the chlorine smell is gone;
4. let the tank dry and fill it with sterilized heated water only when needed; as an alternative possibility, fill the tank with seawater and sterilize with hypochlorite, then neutralize the residual chlorine with sodium thiosulphate (see method in Annex 7).
Repeat the procedure for the equipment to be used in the tanks: aeration tubing, drain valves and suspended traps (see below). A practical procedure is to assemble all small equipment in the new tank, fill with SW and sterilize with hypochlorite: the equipment will be disinfected as a consequence.
Inoculation
To be used as inoculum, the rotifer population must still be in the middle of its log-phase with at least 20% fertility rate (measured as percentage of eggs over total rotifers). Never use rotifers which have already reached the last phase characterised by limited motility, scarce repletion and no egg presence.
In algae/yeast fed tanks, the initial density of inoculum, one of the most important factors in rotifer culture, should be kept at least at 100 animals/ml, with an optimal density of 150-200 animals/ml. High density cultures with artificial feeding need up to 500 rotifers per ml (see below). With an initial density of 200 animals/ml, rotifer density should reach its peak within four to six days at 25°C.

Fig. 30.00 The classic cylindroconical rotifers tank (photo STM Aquatrade)
Tank inoculum may come from large rotifer bags (vertical upscaling), or from other tanks (horizontal upscaling). One day before being harvested to be used as inoculum, rotifers in the tanks should be fed a fertility booster such as Protein Selco® by INVE SA. In order to reduce contamination and culture crash risks, horizontal upscaling should be limited to 7-8 cycles, after which the inoculum should be again taken from large bags.


